Additional Information
| Specialty | |
|---|---|
| Manufacturer | |
| Brand |
PTQ is implanted in the Internal Anal Sphincter (IAS) to treat passive faecal incontinence due to a weak or disrupted internal anal sphincter. The procedure is minimally invasive and technically simple (Ref 1). It can be performed under local anaesthesia in an outpatient setting.
References
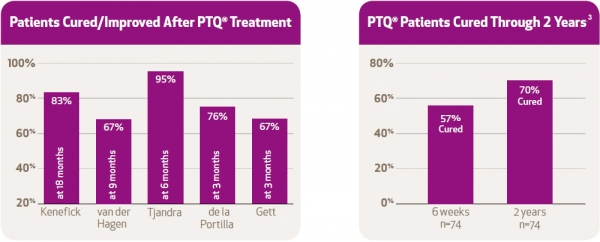
1. PTQ – 2007 – Evaluation of the use of PTQ implants for the treatment of incontinent patients due to internal anal sphincter dysfunction
2. Oliveira, L.C.C., Jorge, J.M.N., Yussuf, S. Habr-Gama, A., Kiss, D., & Cecconello, I. (2009). Anal incontinence improvement after silicone injection may be related to restoration of sphincter asymmetry. Surgical Innovation, 16(2), 155-161.
3. Bartlett,L. & Ho, Y.H.(2009). PTQTM anal implants for the treatment of faecal incontinence. British Journal of Surgery, 96, 1468-1475.
4. Tjandra, J.J., Chan, M.K.Y., & Yeh, H.C.H. (2009). Injectable silicone biomaterial (PTQ™) is more effective than carbon-coated beads (Durasphere®) in treating passive faecal incontinence – A randomised trial. Colorectal Dis., 11, 382-389.
5. Tjandra, J.J., Lim, J.F., Hiscock, R., & Rajendra, P. (2004). Injectable silicone biomaterial for fecal incontinence caused by internal anal sphincter dysfunction is effective. Dis Colon Rectum, 47, 2138-2146.
6. van der Hagen, S.J., van Gemert, W.G., & Baeten, C.G. (2007). PTQ™ Implants in the treatment of faecal soiling. Br J. Surgery, 94, 222-223.
7. de la Portilla, F., Fernández, A., León, E., Rada, R., Cisneros, N., Maldonado, V.H., Vega, J., & Espinoda, E. (2008). Evaluation of the use of PTQ™ implants for the treatment of incontinent patients due to internal anal sphincter dysfunction. Colorectal Dis, 10, 89-94.
8. Kenefick, N.J., Vaizey, C.J., Malouf, A.J., Norton, C.S., Marshall, M., & Kamm, M.A. (2002). Injectable silicone biomaterial for faecal incontinence due to internal anal sphincter dysfunction. Gut, 51, 225-228. 77(Suppl 1), A16.
9. Gaj, F., Trecca, A., & Crispino, P. (2007). “[Efficacy of PTQ agent in the treatment of faecal incontinence].” Chir Ital, 59(3), 355-359.
10. Tjandra, J.J., Tan, J., Lim, F., & Murray-Green, C. (2006). Long-term results of injectable silicone biomaterial for passive fecal incontinence – A randomized trial. Dis Colon Rectum, 49(5), 730-731.
11. Bach, S., Lindsey, I., George, B., Cunningham, C., & McC. Mortensen, N.J. (2004). Implantable silicone for passive fecal incontinence secondary to internal anal sphincter deficiency. Poster ASCRP, Dallas, USA.
| Specialty | |
|---|---|
| Manufacturer | |
| Brand |
The information contained within this website is designed and intended for healthcare professionals only.
Endotherapeutics will not be liable for any actions taken in reliance of the information contained within the website. The information contained within the website does not constitute medical advice.
If you are a patient who requires treatment or management of a medical condition, please click No and you will be redirected to Endo Personal Care.