Improving healthcare with innovative medical technologies
Endotherapeutics improves healthcare by distributing innovative, patient-centric medical technologies accompanied by expert clinical support
Healthcare and Aesthetics technologies to improve your practice or clinic
Improving healthcare for
over 20 years
Endotherapeutics has been committed to improving healthcare by introducing and supporting innovative medical technologies in Australia and New Zealand.
We have a reputation for professionalism, and for delivering the highest levels of customer service and clinical support to all stakeholders.

Looking for an ANZ Business Partner?
Are you a healthcare technologies company looking to work with a reputable, experienced and performance-focused business partner in Australia and New Zealand?
Endotherapeutics specialises in the sales, marketing, regulatory approval and distribution of leading medical technologies.
Explore our range of medical and aesthetics technologies
Endotherapeutics is the exclusive partner of a wide range of leading medical and aesthetic technologies and brands across various specialties.
Catch up with the latest news and announcements from Endotherapeutics
Laborie Transitions Urology and Gastroenterology Sales Model in Australia
Leading diagnostic and therapeutic medical technology company, Laborie Medical Technologies Corp. (Laborie), announced that it has transitioned its Urology (UR) and Gastroenterology (GI) business from a distribution arrangement with Endotherapeutics Pty Ltd to a...
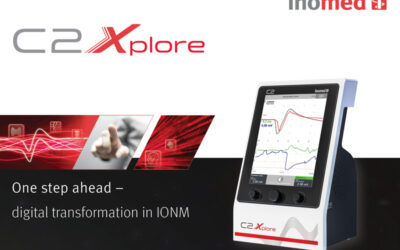
Endotherapeutics partners with inomed Medizintechnik GmbH, adding Intraoperative Neuromonitoring to its OHNS Portfolio
Endotherapeutics is delighted to announce its recent partnership with inomed Medizintechnik GmbH, as their distributor for the C2 Xplore Intraoperative Neuromonitoring solution for OHNS and Thyroid procedures in Australia and New Zealand. We are excited to add the...
Endotherapeutics – the New Home of Dornier MedTech
Endotherapeutics is proud to announce its partnership with Dornier MedTech as their new Exclusive Distributor and Authorised Service Representative for EndoUrology in Australia. Please find the official announcement letter here. We are thrilled to complement our...
Positions Available at Endotherapeutics
No Results Found
The page you requested could not be found. Try refining your search, or use the navigation above to locate the post.
Improve healthcare with Endotherapeutics
Speak to our team today